Good Manufacturing Practices (GMP), find out why they are becoming more and more necessary
Good Manufacturing Practices (GMPs), according to the World Health Organization, offer a generally coded system that ensures that everything produced is consistently produced and checked to certain quality standards.
This system is built to minimize the probability of the occurrence of certain risks usually involved within any pharmaceutical production, which cannot be eliminated later when testing the final product. These risks include: unexpected contamination of products, with damage to people’s health, leading in some cases to death; the problems of incorrect labels affixed to containers that could lead to patients receiving the wrong medicine; insufficient or exaggerated concentration of an ingredient, resulting in ineffective treatment or adverse effects on the patient.
The GMP system concerns itself with and certifies all aspects of production: from raw materials, premises and equipment used, to training and personal hygiene of all workers and so describe the minimum standard that a drug manufacturer must adhere to in all the manufacturing processes.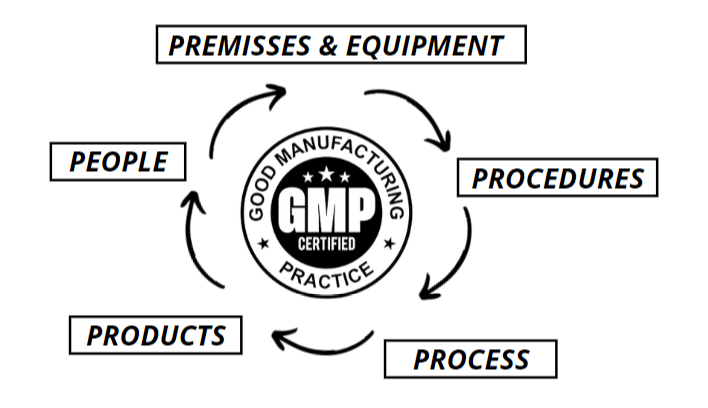
The GMPs are applied to the sense of Title 21 Code of Federal Regulations and can be described, coherently to the Title, in a different connotation in each country.
The World Health Organization (WHO) has established guidelines that must follow good manufacturing practices to be defined as such.
WHO version of GMPs is used by pharmaceutical regulators and the pharmaceutical industry in more than 100 countries around the world and primarily in developing countries.
Other countries, on the other hand, have harmonized their requirements with the GMP directives of other organizations and treaties such as those of the
Association of Southeast Asian Nations (ASEAN), the European Union and the Pharmaceutical Inspection Convention.
The GMP version of the European Union (EU-GMP) imposes similar requirements to the GMP of the WHO, as does the US version of the FDA.
Similar GMPs are also used in other countries such as Australia, Canada, Japan, Saudi Arabia, Singapore, Philippines, Vietnam which have imposed highly specific requirements. In the United Kingdom, within the Medicines Act (1968), “The Orange Guide” covers the majority of the aspects of GMPs. This guide is officially known as the guide to rules and guidelines for pharmaceutical manufacturers and distributors.
Since the first publication by the International Conference on Harmonization (ICH) in 1999, GMPs and the topic of active pharmaceutical ingredients, have remained a constant presence in pharmacological production processes.
In fact, even with the subsequent and related improvements, the GMPs are applied to date in all ICH signatory countries and commercial groups (EU, Japan and United States) and in all other countries (e.g. Australia, Canada, Singapore) that adopt the guidelines ICH for the production and control of active raw materials.
As mentioned above, there are specifications that each Country affixes to the GMPs. In the United States, for example, they are regulated by the Food and Drug Administration (FDA) and the regulations use the phrase “Current Good Manufacturing Practice” (CGMP) to describe these guidelines. Courts can find that a product has failed if its manufacturing process has not been performed to industry standards, even if there is no specific regulatory requirement.
The European Medicines Agency (EMA), besides, organizes and manages inspections to verify compliance with these standards and plays a key role in combining the production activities of companies with GMPs at European level. Any manufacturer of medicines (that are destined for the European market) will therefore have to comply with EU GMPs.
In general, the Good Manufacturing Practices certify that medicines are consistently of high quality maintained over time and space; that medicines are suitable for their intended use and that they meet the requirements to be marketed or clinically tested.
GMPs are based on the detailed description of the manufacturing procedures, which are essential for each of the processes that could affect the quality of the finished product. So GMPs can be defined as a system that supports companies to provide documented evidence that correct procedures are consistently followed at every stage of the manufacturing process, every time a product is made.
Why is it important to follow the GMP guidelines? Originating within the pharmaceutical context, Good Manufacturing Practices prevent poor quality drugs from creating a danger to health and, at the same time, a waste of money for both States and individual consumers.
A medicine that is not manufactured according to GMP guidelines may contain alternatively added substances during its manufacturing process.
When these can be present in significant quantities, they can cause sometimes irrevocable damage; while when their quantity is reduced, the effect will in any case be negative as the medicine will not have the expected effect.
Most countries, at these days, only accept the import and sale of medicines that have been manufactured according to internationally recognized GMPs, avoiding internal issues, and it is estimated that these restrictions will grow over the next few years.
Therefore, to promote their own export of pharmaceutical products, different countries will make GMPs compulsory internally on all pharmaceutical production and will form real tasks of inspection of GMP requirements, in order to be able to freely carry out the desired exchanges.
This is where Solaris comes in, being able to transfer information and equipment to customers in line with GMP guidelines means being able to help the customer achieve the desired production.
The continuous training of Solaris staff on how to best follow the GMP directives allows the company to feel able to satisfy and respond in the best way to the needs of customers, always providing professional and detailed information.
In collaboration with PAT Way Solutions – Process Automation Technologies, Best Manufacturing Practices